
SL Paper 1
Which represents a p orbital?
Markscheme
C
Examiners report
Which is correct for the line emission spectrum for hydrogen?
A. Line M has a higher energy than line N.
B. Line N has a lower frequency than line M.
C. Line M has a longer wavelength than line N.
D. Lines converge at lower energy.
Markscheme
C
Examiners report
What is the ground state electron configuration of an atom of chromium, Cr (Z = 24)?
A. [Ar]3d6
B. [Ar]4s23d4
C. [Ar]4s13d5
D. [Ar]4s24p4
Markscheme
C
Examiners report
A large number of the candidates had ground state configuration of Cr as 4s2 3d4 rather than 4s1 3d5
Which electron transition emits radiation of the longest wavelength?
Markscheme
C
Examiners report
Which represents the shape of an s atomic orbital?
Markscheme
A
Examiners report
Which is correct for ?
Markscheme
D
Examiners report
A well answered question. 88 % of the candidates deduced the correct numbers of protons, neutrons and electrons in the sulfide ion.
What is the condensed electron configuration of the Fe2+ ion?
A. [Ar]3d6
B. [Ar]3d44s2
C. [Ar]3d54s1
D. [Ar]3d64s2
Markscheme
A
Examiners report
What is the number of protons and the number of neutrons in 131I?
Markscheme
A
Examiners report
What is represented by A in ?
A. Number of electrons
B. Number of neutrons
C. Number of nucleons
D. Number of protons
Markscheme
C
Examiners report
Naturally occurring gallium consists of the isotopes 71Ga and 69Ga. What is the approximate percentage abundance of 69Ga?
Mr (Ga) = 69.72.
A. 40 %
B. 50 %
C. 60 %
D. 75 %
Markscheme
C
Examiners report
Which technique is used to detect the isotopes of an element?
A. Mass spectrometry
B. Infrared spectroscopy
C. Titration
D. Recrystallization
Markscheme
A
Examiners report
What is the relative molecular mass of bromine, according to the following mass spectrum?
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
A.
B.
C.
D.
Markscheme
A
Examiners report
Which is the electron configuration of a chromium atom in the ground state?
A. [Ne]3s23p64s13d4
B. [Ar]3d3
C. 1s22s22p63s23p64s23d4
D. [Ar]4s13d5
Markscheme
D
Examiners report
What is the maximum number of electrons that can occupy the 4th main energy level in an atom?
A.
B.
C.
D.
Markscheme
D
Examiners report
Poorly answered questions from candidates in general. Only 38% of candidates could state the maximum number of electrons in the 4th principal energy level is 32, with the most common incorrect answer being 18.
Which statements are correct for the emission spectrum of hydrogen?
I. The lines converge at higher frequencies.
II. Electron transitions to n = 2 are responsible for lines in the visible region.
III. Lines are produced when electrons move from lower to higher energy levels.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
In which set do all the species contain more electrons than neutrons?
A. 14N, 16O, 11C
B. 14N, 16O, 11C4–
C. 14N3–, 16O2–, 11C
D. 14N3–, 16O2–, 11C4+
Markscheme
C
Examiners report
What is the relative atomic mass, , of an element with this mass spectrum?
A.
B.
C.
D.
Markscheme
B
Examiners report
Well answered question with more than 70% of candidates able to find the RAM of an element from isotope relative abundances.
Which are correct statements about the emission spectrum of hydrogen in the visible region?
I. The red line has a lower energy than the blue line.
II. The lines converge at longer wavelength.
III. The frequency of the blue line is greater than the frequency of the red line.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which of the following is the electron configuration of a metallic element?
A. [Ne] 3s2 3p2
B. [Ne] 3s2 3p4
C. [Ne] 3s2 3p6 3d3 4s2
D. [Ne] 3s2 3p6 3d10 4s2 4p5
Markscheme
C
Examiners report
Bromine consists of two stable isotopes that exist in approximately a 1 : 1 ratio. The relative atomic mass, Ar, of bromine is 79.90. Which are the stable isotopes of bromine?
A. 79Br and 81Br
B. 80Br and 81Br
C. 78Br and 80Br
D. 79Br and 80Br
Markscheme
A
Examiners report
Almost 56 % of candidates could find a 1:1 ratio as an average of 2 items, however many had 79Br and 80Br with an average mass of 79.90
What does represent?
A. An ion with 12 protons and 24 neutrons
B. An ion with 14 protons and 24 neutrons
C. An ion with 12 protons and 12 neutrons
D. An ion with 12 protons and 22 neutrons
Markscheme
C
Examiners report
Which statement about 56Fe3+ and 54Fe2+ is correct?
A. Both have the same numbers of protons and electrons.
B. Both have the same number of protons.
C. Both have the same number of neutrons.
D. Both have the same numbers of protons and neutrons.
Markscheme
B
Examiners report
The full electron configuration of an element is:
1s22s22p63s23p2
To which group and period does the element belong?
Markscheme
D
Examiners report
What is the relative atomic mass of an element with the following mass spectrum?
A. 23
B. 24
C. 25
D. 28
Markscheme
B
Examiners report
69% of the candidates determined the relative atomic mass of the element from its mass spectrum.
How are emission spectra formed?
A. Photons are absorbed when promoted electrons return to a lower energy level.
B. Photons are absorbed when electrons are promoted to a higher energy level.
C. Photons are emitted when electrons are promoted to a higher energy level.
D. Photons are emitted when promoted electrons return to a lower energy level.
Markscheme
D
Examiners report
What is represented by “2−” in ?
A. loss of electron
B. gain of electron
C. loss of proton
D. gain of proton
Markscheme
B
Examiners report
Which experimental results support the theory that electrons exist in discrete energy levels?
A. 1H NMR
B. X-ray diffraction pattern
C. Emission spectra
D. IR spectra
Markscheme
C
Examiners report
A well answered question. 84% of the candidates identified emission spectra as the experimental results that support the theory that electrons exist in discrete energy levels.
What is the composition of the nucleus of 26Mg?
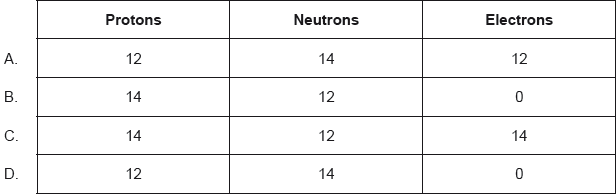
Markscheme
D
Examiners report
How many p-orbitals are occupied in a phosphorus atom?
A. 2
B. 3
C. 5
D. 6
Markscheme
D
Examiners report
Which electron transition in the hydrogen atom emission spectrum emits radiation with the longest wavelength?
A. n = 2 → n = 1
B. n = 1 → n = 2
C. n = 4 → n = 1
D. n = 3 → n = 2
Markscheme
D
Examiners report
What is the maximum number of electrons that can occupy a p-orbital?
A. 2
B. 3
C. 6
D. 8
Markscheme
A
Examiners report
Question 6 was poorly answered as it asked for the number of electrons in a p orbital. Very few students gave the correct answer of 2. The majority chose answer C (6) for the maximum number of electrons that can occupy a p-orbital, rather than A (2). It appears candidates reflexively conflated p-orbitals with the entire p subshell in any given period.
Which transition in the hydrogen atom emits visible light?
A. n = 1 to n = 2
B. n = 2 to n = 3
C. n = 2 to n = 1
D. n = 3 to n = 2
Markscheme
D
Examiners report
This question discriminated well between high scoring and low scoring candidates. 68% of the candidates chose the correct transition that emits visible light (n = 3 to n = 2). The most commonly chosen distractor C was the only other option that involved emission.
Which shows the number of subatomic particles in 31P3−?
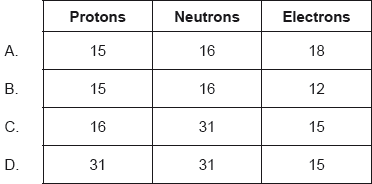
Markscheme
A